
Introduction
In museum environments it is desirable that all construction materials which are to be used near museum objects do not emit reactive pollutants. We describe a new method to collect emitted organic components from an emission test chamber. The method is fast and does not use organic solvents. It is possible to detect acetic acid down to approximately 5-10 ppb.
Emission test chamber
A specimen of the material to be tested is placed in a stainless steel chamber with a volume of 227 liters. The chamber is flushed with a constant flow of purified and conditioned air. Normal test conditions are 23°C, 45% RH, and an air flow through the system between 0.01 - 0.2 m3/h. The chamber set-up is described elsewhere [Ryhl-Svendsen, 1999].
Sampling media
Sampling is performed using Solid Phase MicroExtraction (SPME)(Supelco). SPME consists of a fused silica fibre coated with a highly adsorbant polymer which is fitted in a syringe-like holder. The polarity of the needle coating is chosen to match the polarity of the components of interest.

Fig. 1: The SPME holder with the fiber exposed (left). The size can be compared with the pencil.
Sampling is performed by exposing the fiber to the air leaving the chamber. Analysis of collected components was performed by gas chromatography/mass spectroscopy (GC/MS) using the injector to desorb the components collected. No organic solvents are used during analysis, and the time used from start of sampling to analysis results is 35 minutes.

Fig. 2: The sampling setup, connected to the chamber by teflon tubings. The SPME fibre is in position ready for sampling, at the white Omnifit teflon holder next to the pump.
The air sample is sucked out of the test chamber exhaust manifold by a personal sampling pump, past a teflon holder with the SPME fiber exposed (Fig. 2). Fifteen minutes was found to be a sufficient sampling time, during this less than 4 liters of air were sampled.
Calibration
The fiber was exposed to a range of standard concentrations of acetic acid in air, from 5 - 400 ppb (approx. 12 - 1000 µg/m3). There is a linear relationship between concentrations and chromatogram peak area within this range.
Emission rate vs. concentration
The resulting chromatographic peaks are proportional to the mass of the given component collected on the SPME fiber. This can be converted to concentration. However, when making a comparative study of a range of different materials the emission rate of the pollutant from the material surface is a more useful measure.
The general equation for the area specific emission rate (ER) of a material is:
ER = (C x F) / A
Where:
ER = area specific emission rate [µg/m2h]
C = chamber concentration [µg/m3]
F = air flow rate [m3/h]
A = surface area of material [m2]
Three examples of tested materials
1. Drawer for coin collections, made of Masonite and sycamore wood
A drawer from the storage vault of the National Museum's Coin and Medal Collection was tested. Lead coins kept in these drawers were found to corrode heavily. At the time of the emission test the drawer was about 12 years old.
Emission of acetic acid from one drawer = (930 µg/m3 x 0.102 m3/h) = 95 µg/h
Taking the surface area into account:
ER = (930 µg/m3 x 0.102 m3/h) / 0.550 m3= 173 µg/m2h
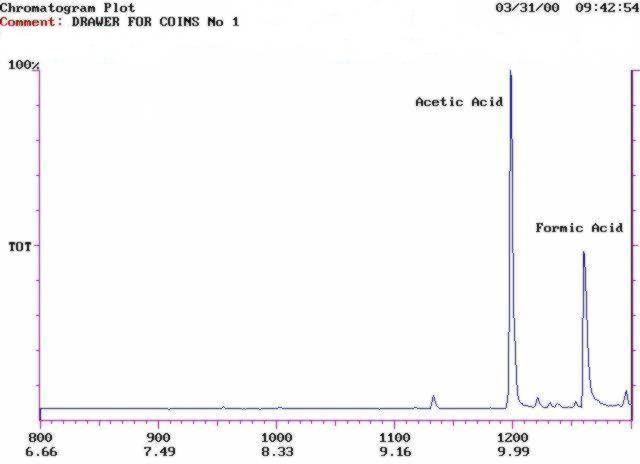
Fig. 3: Chromatogram of emission from the wooden drawer. The acetic acid emission was measured quantitatively but a formic acid peak is also visible.
2. Cellulose acetate photographic negative
A cellulose acetate negative is of course not a construction material. However, it is an example of an object which acts as a pollution source, which is a difficult situation to cope with in museum environments. While bad construction materials can be discarded, museum objects or archival materials must be accepted within the collection. The air quality problems that may arise must be dealt with in other ways. This negative was chosen as an example of such objects. Cellulose acetate is found in a large number of modern cultural objects, unfortunately the material deteriorates, releasing acetic acid in large quantities. This is a well known problem for collections of photographic materials where it is known as "the vinegar syndrome". The tested negative is from the late 1950's, and shows signs of degradation.
Emission of one negative = (924 µg/m3 x 0.096 m3/h) = 89 µg/h
Taking the surface area into account:
ER = (924 µg/m3 x 0.096 m3/h) / 0.0190 m3 = 4685 µg/ m2h
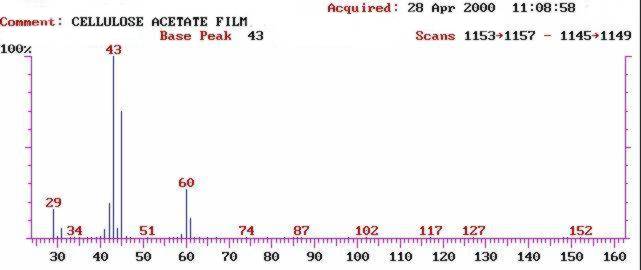
Fig. 4: Mass spectrum of the acetic acid peak from a cellulose acetate negative emission test. Together with the retention time from the GC chromatogram, a mass spectrum gives a high confidence in identification of compounds
The two examples above illustrate well the usefulness of the emission rate concept. At first it seems that the two items are equal polluters, as they produce almost equal concentrations of acetic acid in the test chamber. However, taking the differences in their surface area into account (the negative is much smaller than the drawer) the cellulose acetate releases tremendous amounts of acid compared to the wood of the drawer. Actually the emission from the negative is so high that the base is deforming and shrinking, due to the deterioration process.
3. Silicone sealant (acid curing)
By monitoring the emission over time for a material, it is possible to estimate the time needed for the material to offgass until the emission rate of pollutants reaches a low enough level that the material can be considered safe.
As an example of this the emission profile for an acid curing silicone sealant (Bostik Silicone 2680) is shown below (fig. 5). The sealant was exposed in the chamber as a 0.5 m joint cast into an aluminium U-profile.
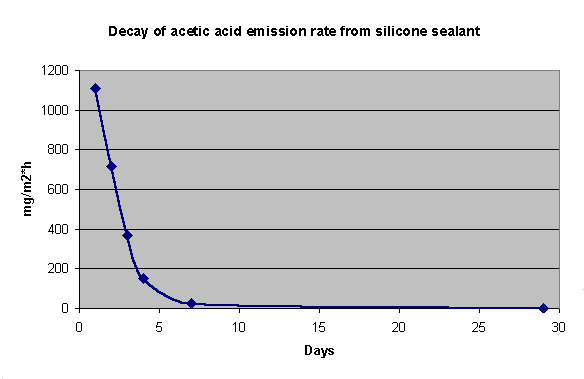
Fig. 5: Emission profile of Bostik 2680 silicone sealant over 29 days.
Concluding remarks
SPME is a solvent free and cheap sampling method for acetic acid. Analysis is fast, and detection limit is in the low ppb level. The method seems promising also for formic acid: this will be the subject for further work. In general, SPME is useful for a large range of VOC's.
Materials and method used
SPME: 85µ polyacrylate (cat. no. 57304) from Supelco
Sampling setup: Omnifit Universal Septum (teflon) injector, nr. 3301 connected to a SKC model 222-3 personel pump by teflon tubing.
Analysis: 20 m J&W DB-wax column, 0.18mm id. 0.3µ coating, part nr. 121-7023.
Varian 3400 GC/MS
GC Method:
| Start (°C) | End (°C) | Rate (°C/min) | Time (min) |
| 30 | 30 | 0.0 | 3.0 |
| 30 | 230 | 15.0 | 13.33 |
| 230 | 230 | 0.0 | 3.0 |
Related links
The National Museum of Denmark, Conservation Dept.
References
Morten Ryhl-Svendsen: "The pleasures and pains of emission chambers - the construction of an emission test chamber at the National Museum of Denmark". In (Eds: Agnes W. Brokerhof & Lorraine Gibson) Indoor Air Pollution: Detection and Prevention, Presentation Abstracts and Additional Notes. Instituut Collectie Nederland, Amsterdam, The Nederlands, 26-27 August 1999.
Also on-line: http://iaq.dk/iap/iap1999/1999_10.htm
Morten Ryhl-Svendsen
The School of Conservation, The Royal Danish Academy of Fine Arts
Esplanaden 34, DK-1263 Copenhagen K, Denmark
morten@ryhl.dk
Jens Glastrup
The National Museum of Denmark, Conservation Department
Brede, DK-2800 Kgs. Lyngby, Denmark
jens.glastrup@natmus.dk
[ Page up ] [ IAP Group homepage ] [ Main IAQ in Museums homepage ] [ Search site ]
Indoor Air Quality in Museums and Archives © 2000