|
Exhibit cases are usually necessary to protect cultural objects from theft as well as from dust and mechanical damage. Based on emission measurements in exhibit cases of the museum "Grünes Gewölbe" in Dresden, Germany, it can be shown that atmospheric conditions inside the cases endanger the exhibits in show cases especially with low air exchange rates (air exchange rate < 0.1 h-1).

Figure 1: Detail from the "Mogul's Court"
|
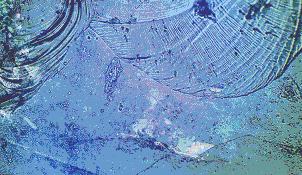
Figure 2: Polarizing photomicrograph
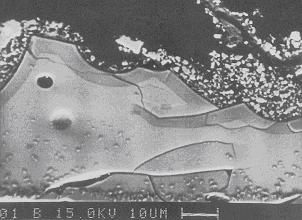
Figure 3: Scanning electron micrograph
|
Glass corrosion
The collection in Dresden contains many glass and enamel objects of art, the most famous one is the so called "Hofstaat des Grossmoguls" from the goldsmiths J.M. and G.F. Dinglinger (1701-08). This object consists substantially of noble metals and applied opaque and translucent enamels (Fig. 1). Conservation studies demonstrate that translucent enamels are damaged in particular indicating cracks and spallings (Fig. 2). Further investigations using electron probe microanalysis (EPMA) and energy dispersive X-ray spectroscopy (EDS) present a correlation between cracks in the material and gel coats (Fig. 3). This gel formation is caused by action of acid (hydrolysis) giving rise to a preferential leaching of alkali ions (e.g. Na, K) and their replacement by hydrogen ions. The silicates are converted into a hydrated silica network which reacts very sensitive concerning changing temperature and humidity. In comparison to opaque enamels translucent enamels contain alkali-silicate glasses with small amounts of colouring oxides. As opaque enamels consist of lead silica glass with high amount of SnO2 (opacifier) they show an increased resistance against hydrolysis (Table 1).
Table 1: Compositions of different enamels
|
enamel | main components | minor comp. | coloring comp. | |
| SiO2 | Na2O | PbO | SnO2 | K2O | CaO | Fe2O3 | CuO | |
blue, transluzent | 60-68 | 18-22 | - | - | 2-6.5 | 0.2-1 | 0.3-3 | 2.5-8.5 | |
green, transluzent | 59-70 | 15-21 | - | - | 2.5-5.5 | 0.5-3.5 | 1.5-4.5 | 6-11 | |
green, opaque | 39-48 | 7.5-15 | 15-22 | 14-28 | 1.5-3.5 | 0.5-2 | - | 0.5-3 | |
white, opaque | 39-49 | 8.5-12.5 | 20-27 | 20-28 | 1-2 | 0.2-2.5 | - | - |
Emission measurements
It is known that wood is a source of organic acids, especially formic and acetic acid. Although many research is done on VOC-emission measurements from materials that are used indoors, poor data exist for the emission behaviour of these organic acids, due to the fact that they are not caught with the common analytical methods for VOC-determination. Two methods for the investigation of organic acids in exhibit cases were tested. The first one is a standard method for VOC-determination (thermal desorption, TDS). The second one used derivatisation of the acids with pentafluorobenzyl bromide (pfbbr) after sampling and solvent extraction. Acetonitrile has the advantage to be one of the most suitable solvents for the acid derivatisation with pfbbr. Only two steps of sample preparation are necessary to get an extract, that is analysable with a GC/MS-system. The resulting derivatives are stable and amenable to gas chromatographic separation, after that mass spectroscopy detection greatly im-proves the selectivity and sensitivity of the method. Reliable quantitation limits with tenax sampling are in lower µg m-3 range, with a standard error estimated < 5 % apart from formic acid (Tab. 2). With TDS detection limits in the lower µg m-3 range are realisable with the exception of formic and acetic acid. They are caused by high blank values. The formic acid is not determinable with TDS in the described way. The standard error of determination is higher for the lower acids. Caused by the considerable influence of the free carboxylic group, interactions with active points of the analytical system can take place.
Table 2: Detection and quantification limits of C1 - C6 fatty acid determination (thermal desorption, TDS, in comparison with a derivatisation method, Derivat., sample volume: 1l)
| DL [µg m-3] | QL [µg m-3] | RSD [%]
| |
| TDS | Derivat. | TDS | Derivat. | TDS
Ci = 500 µg m-3 | Derivat.
Ci = 250 µg m-3 | |
formic acid | n.a. | 10* | n.a. | 30* | - | 15.3 | |
acetic acid | 26* | 13* | 78* | 40* | 18 | 5.5 | |
propanoic acid | 8 | 0.3 | 23 | 1 | 11 | 4.7 | |
butanoic acid | 2 | 0.5 | 6 | 1.5 | 8 | 4.1 | |
valeric acid | 2 | 0.4 | 7 | 1.2 | 9 | 4.4 | |
hexanoic acid | 4 | 0.3 | 12 | 0.9 | 11 | 4.2 | |
|
DL: detection limit; QL: quantitation limit; RSD: relative standard deviation
n.a.: not analysable; *: caused by the blank value
 Figure 4: 0.02 m3 Emission test chamber
Figure 4: 0.02 m3 Emission test chamber
Additionally several components of exhibit cases were investigated in emission test chambers (Fig. 4). The acid emissions were investigated in the range from formic acid to hexanoic acid. Sampling was carried out on Tenax tubes, using a Gilian PP1-Ex low flow sampler. Generally air volumes of about l l were sampled with a flow rate of 100 ml min-1 for the adsorption tubes. The analysis was performed with thermal desorption (Gerstel TDS-2) in combination with gas chromatography (HP-GC 5890 II plus) and mass spectrometry (HP-MSD 5972). The fluid extraction of the Tenax tubes followed by a derivatisation with pentafluorobenzyl bromide was done with acetonitrile. The derivatisation procedure is based on methods described by Brill et al. The analysis was carried out with the same technical equipment using a cold injection system and a RTX-200 capillary column.
Results
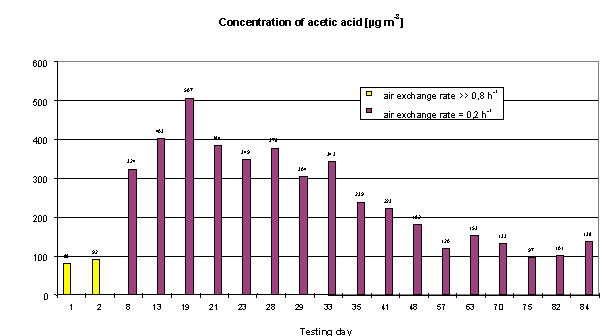
Comparable measurements with both methods in show cases revealed organic acids, especially acetic acid in concentrations up to 3 mg m-3 (Fig. 5). Lower concentrations of hexanoic acid were analysed with these methods, too (Tab. 3). Obviously those concentrations of organic acids might be a problem for air quality in closed show cases in particular. The acid emission as well as the emission of other organic compounds such as alpha-pinene was caused by wood-based parts inside the show case. Additional measurements of different wood materials analysed considerable emissions of formic and acetic acid (Tab. 4). Acid emissions, especially acetic acid plays an important role during investigations of different materials for exhibit cases and should be analysed and considered together with other VOC (Fig. 6).
Figure 5: Chromatogram of Tenax-sampling in a show case (sample volume 1l)
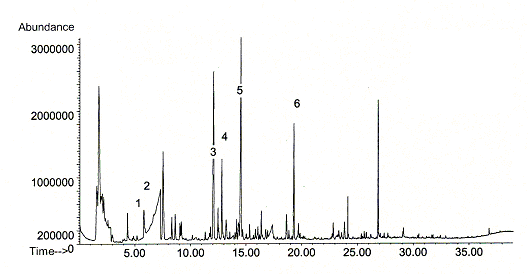
Table 3: Concentration of Volatile Organic Compounds (VOC) in a show case (1)
| VOC |
[µg m-3] |
|
acetic acid | 3000 | |
hexanoic acid | 30 | |
butanol | 24 | |
propanoic acid | 15 | |
toluene | 12 | |
hexanal | 11 | |
furfural | 10 | |
camphene | 10 | |
32 further VOC (1-10 µg m-3 total amount) | 93 | |
Total VOC | 3205
|
Discussion
The described sample collection and derivatisation method followed by GC/MS-analysis presents a sensitive and practicable method for analysis of C2-C6 fatty acids in air samples. In comparison to TDS the fluid extraction allows multiple analysis runs, better detection limits and a higher precision. The additional information about formic acid concentrations is necessary for the interpretation of air quality. Another advantage of the derivatisation technique is the selectivity for acid compounds.
In addition the investigations show that for a good preservation of fragile objects in exhibit cases sufficient ventilation is absolutely necessary. Furthermore the investigation of single components of show cases in emission test chambers demonstrate that it is important to use low-emission materials for the construction of exhibit cases.
Table 4: Sample concentrations [µg m-3] of volatile fatty acids
|
|
show case 2
|
show case 3
|
test chamber with
chipboard (10th day)
|
|
|
TDS
|
Derivat.
|
TDS
|
Derivat.
|
TDS
|
Derivat.
|
|
formic acid
|
n.a.
|
583
|
n.a.
|
280
|
n.a.
|
187
|
|
acetic acid
|
1177
|
1555
|
475
|
510
|
499
|
555
|
|
hexanoic acid
|
9*
|
56
|
< QL
|
15
|
< QL
|
7
|
QL: quantitation limit; n.a.: not analysable;
*: quantified with response factor of cyclodecan
Figure 6: Concentrations of acetic acid in a model show case
References
J.H. Brill, B.A. Narayanan, J.P. McCormick, Selective determination of pentafluorobenzyl ester de-rivatives of carboxylic acids by GC using microwave plasma and mass selective detection, Appl. Spectrosc. Vol. 45, pp. 1617 - 1620 (1991).
S. Lange, O. Wilke, D. Broedner, O. Jann, Measuring the emission behaviour of organic acids from wooden products in test chambers, Proceedings of the 8th International Conference on Indoor Air and Climate- Indoor Air 1999, Edinburgh, Scotland, UK, Vol. 5, pp. 143 - 148 (1999).
O. Jann, O. Wilke, D. Brödner, U. Schneider, S. Marten, Die Umgebungsluft von Exponaten - Belastung durch flüchtige organische Verbindungen, Restauro 6, pp. 428 - 432 (2000).
W. Müller, K. Adam, D. Kruschke, C. Neelmeijer, M. Mäder, Welche Ursachen haben die Schäden an Emailkunstwerken? Restauro 6, pp. 414 - 418 (2000).
Author to whom correspondence may be addressed:
Oliver Hahn
Bundesanstalt für Materialforschung und -prüfung (BAM)(Federal Institute for Materials Research and Testing)
Unter den Eichen 44-46
D-12203 Berlin
Germany
E-mail: oliver.hahn@bam.de
|