Calcareous objects in natural history collections such as sea shells and bird's eggs, can be attacked by volatile organic compounds (VOCs), especially formaldehyde and acetic acid. This damaging phenomenon, often referred to as "Byne's disease" or rather "Bynes efflorescence", is especially a problem in natural history collections that are kept in original oak wood storage cabinets. Laboratory studies by Brokerhof and van Bommel (1996) and by Tetreault et al. (1998) into the degradation of calcareous materials and metals by acetic acid vapours have shown the likely existence of a so called "Acceptable Damage Concentration" (ADC). As long as the concentration of acetic acid in the air remains below a certain level, the damage caused to the exposed material is so small that we can accept it. The experiments indicate that even at long term exposures the damage remains acceptable as long as the ADC is not exceeded. What is "acceptable" depends on the function and the application of the object. Damage that cannot be seen with the naked eye may be inacceptable when the same object is studied under the microscope.
The model studies with acetic acid carried out so far provide some insight into the process of degradation, yet it is still unknown what safe levels for exposure are in museum practice. One of the reasons for this is that acetic acid does not act alone, yet the interaction with other factors such as other indoor pollutants, relative humidity, temperature and salts present in the materials are not yet understood. However, it has been established that degradation can be reduced by keeping the acetic acid concentration and the relative humidity as low as possible.
The first options for preventive conservation is removal of the source, but this is unethical if the oak cabinet is an integral part of the shell or egg collection. The second option would be reducing the RH below 40%, which in practice is not always possible either. The best option would be to reduce the acid concentration. This can be accomplished by 1) increased ventilation to avoid build-up of high concentrations of indoor pollutants, 2) filtration of the air inside the storage cabinet or area and 3) scavanging the pollutants by placing sorbents close to the objects. For the old wooden cabinets the last method is the most suitable.
Method
In the laboratory, eggshells were exposed, for a period of 8 months, to a 100 ppm concentration of acetic acid at 70-80% RH in the absence and presence of sorbents. Three pieces of eggshells with known weights were placed on a glass Petri dish which was suspended with nylon wire in a glass jar. The sorbents were placed under the Petri dishes. A separate Petri dish was prepared for each sorbent (figure 1). Two controls and one blank were kept. One set of eggshells was kept unexposed in the climate chamber ("control"), another was exposed to the same climatic conditions but without acetic acid (0 ppm/70-80% RH, "zero exposure"). As a comparison one set of eggshells was exposed to 100 ppm/70-80% RH without sorbent ("blank exposure"). Each month the samples were weighed.
The main component of eggshell is calcite (hexagonal CaCO3) which reacts with acetic acid (CH3COOH) to produce calcium acetate monohydrate (Ca(OOCCH3)2 x H2O) according to the reaction:
(1) CaCO3 + 2 CH3COOH -> Ca(OOCCH3)2·H2O + CO2
Calcium acetate monohydrate (mw 176) is heavier than calcium carbonate (mw 100), thus the weight of the eggshell increases after reaction. Damage to the calcareous surface can therefore be quantified by measuring the associated weight change. Since the reaction takes place at the surface of the material, ideally, weight changes should be related to the available surface. In these experiments, the eggshell pieces were flat and thin and so, weight changes were related to the initial total weights of the samples. To be able to compare the weight increases of different pieces of eggshell, weight gains (Wt), which are the weight of the eggshells after exposure minus the initial weight of the eggshells (Wt - Wt0), were expressed as a percentage of the initial sample weights (Wt0):
(2)
Wt (%) = {(Wt - Wt0) / Wt0} x 100
Sorbents
Different acid sorbents were tested:
Experimental
Three successive experiments were carried out to investigate the efficacy of the different sorbents, their capacity and their influence on the RH. In the first experiment, eggshells were exposed to the acidic environment in the presence of copy paper (1), KOH-paper (2) and Vokes filter material (3). Each month, the acetic acid concentration in the environment was replenished and the sorbents were replaced to study the protective potential of the sorbents. In the second experiment the sorption potential of zeolites (4) and Microchamber paper (5) was studied as well as the time taken to saturate the sorbents at the chosen concentration. The effect of the various sorbents on the relative humidity inside the jars was also determined in a separate experiment. Here, the eggshells were replaced by small hygrometers, placed on the Petri dishes, and RH was measured over a period of 4 weeks.
Results and Discussion
Observations
Upon exposure of the eggshells to 100 ppm acetic acid at high RH, 70 - 80 %, wetting of the calcareous surface was observed. After the eggshells darkened, due to the presence of moisture, small water droplets were formed on the surface and eventually drops of water collected on the bottom of the Petri dish. The calcium acetate solution forms in this surface film of moisture. Only after the moisture had evaporated, salt crystals occured on the surface and on the bottom of the dish. A high RH alone did not wet the eggshell surface. This can be explained by the fact that calcium acetate is hygroscopic and contains crystal water.
Another interesting observation was that in the presence of the sorbents, the eggshells became moist, while the sorbents became wet. Especially the copy paper which became saturated with water and developed mould growth. Eggshells exposed only to a high RH developed mould growth as well, a high acetic acid concentration inhibited mould growth on the eggshells at high RH.
Weight change
Figure 2 shows the cumulative weight change of the eggshells in time in the presence and absence of copy paper, KOH impregnated filter paper and Vokes activated charcoal with KOH filter material. Without sorbent the weight of the eggshells exposed to 100 ppm HAc, "blank exposure", shows a linear increase in time resulting in a cumulative weight change of 30.4% in 6 months. The rate of weight change, or the rate of degradation, can be expressed by the slope of the line, which for the blank was 5.1 %/month. The control shows no change. With a single Vokes filter, the rate of degradation is reduced to 1.1 %/month; with double quantity 0.5 %/month, roughly half the rate. KOH-impregnated filter paper has the same effect as the two Vokes filters, reducing the rate to 0.5 %/month. Copy paper has some effect, but it was not as pronounced as the other materials, reducing the rate of degradation to an average of 3.0 %/month. Considering that the amount of base in the copy paper ("alkaline reserve") is comparable to the amount in the KOH impregnated filter paper, the difference in protective potential between the two can be explained by the fact that KOH is a stronger base than CaCO3. Acid will prefer to react with KOH whereas CaCO3 in paper will only compete with the eggshells. The amount of base in the double Vokes filter is less than that in the KOH impregnated filter paper, yet the effect is the same due to sorption by the charcoal.
In the second experiment the amount of copy paper was increased, and two other materials, zeolite molecular sieve and MicroChamber zeolite containing sorbent paper, were tested. To study the capacity of the sorbents, they were not replaced during the experiment. Figure 3 shows the cumulative weight change during an 8 month exposure. The unprotected eggshells show a linear weight increase in time with a rate of 4.9 %/month, which is in agreement with the first experiment. The eggshells that are exposed to acid in the presence of sorbents that are changed each month also show a linear weight increase. In contrast to the first experiment, increasing the amount of copy paper from 10 to 18 resulted in a strong linear reduction of the degradation rate (0.9 %/month). Zeolite molecular sieve, replaced each month, slows down the weight change to 1.8 %/month. When not replaced, the same amount of zeolite loses some of its sorption efficiency after 1.5 month. During the first month and a half the two zeolite lines have approximately the same slope (1.7-1.8 %/month). After that, the slope of the unchanged zeolite line increases corresponding to an increased weight change of 2.9 %/month. After which time the response remained linear indicating that saturation of the zeolite had not yet been reached. MicroChamber paper shows an increasing rate of degradation in time. During the first month of exposure the rate of weight change of the eggshells is comparable to that of the zeolite (1.4 %/month), then it increases to a rate of 5.1 %/month during the last 4 months, which is comparable to the weight change of unprotected eggshells. This result suggests that the saturation level of the MicroChamber paper has been reached. The amount of zeolite in MicroChamber paper is unknown, but on a weight basis it is less efficient than Vokes, which gave a similar effect when less sorbent material was used. The two lines of the single and double quantity of Vokes filter material also show an exponential weight change in time when they are not replaced. The two lines begin with a a slope of 1.0-1.2 %/month. After 1.5 month the slope corresponding to the single Voxes filter increases to 2.9 %/month over the next 2.5 month, where it then increases further to an average rate of 4.6 %/month over the last 4 months. This rate of weight increase is almost similar to the unprotected eggshells, suggesting saturation of the sorbent. The slope corresponding to the two Voxes filters begins to increase after 3 months with an average rate of 2.9 %/month for the next 4 months, ending with a rate of 6.3 %/month for the last month.
Relative humidity in the jars
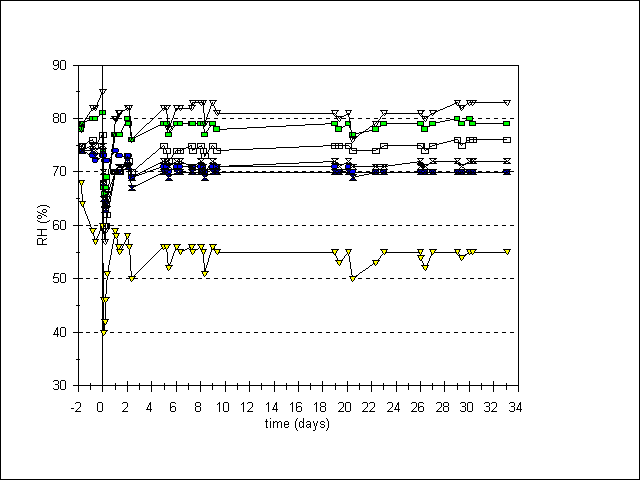
The weight measurements do not clarify what slows down the degradation of the eggshells: sorption of acid, sorption of moisture or of both. The wet paper clearly indicates sorption of moisture, but the quantity of water in the jar should be large enough to keep the RH in the jar at 70-80%. The results of the exposure of small hygrometers show that the effect of the sorbents on the RH is negligible, thus the effect of the sorbents is based solely on scavanging acid (figure 4).
Conclusions
The results of the first experiment indicate that sorbents provide protection against degradation of calcareous surfaces in an acetic acid environment. Sorbents containing a strong base (KOH), that have a preference to react with acid, perform better than calcium carbonate containing material, that reacts with acid in competition with the eggshells. Increasing the amount of sorbent results in a reduction of the degradation. This is confirmed in the second experiment with the increased amount of copy paper. Since the sorption process takes place at the surface of the sorbent, the total surface of the used materials is more important than the total amount of sorbent. When not replaced in time, sorbents become saturated and lose their protective potential. The effect of the sorbents is based on the actual reduction of the acid concentration in the enclosed space. The influence of the sorbents on the relative humidity is negligible.
From the point of view of practicality and economy, KOH-impregnated filter paper is the best option for the protection of objects against acetic acid. Vokes charcoal/KOH filter material proves to be a suitable acid sorbent with the additional advantage of containing a general sorbent to scavange other VOCs as well.
Translated to practical application of sorbents in museums, the results of this study imply that acid sorbents could be used as liners of the drawers and shelves in old oak cabinets. Copy paper or self-made KOH-impregnated filter paper can be used as low cost solutions. An advantage of thr filter papers is that they are white and that saturation of the sorbents can be monitored with pH indicators. Applying a drop of colour indicator, such as bromo thymol blue (blue at pH>7.6, yellow at pH<6.0), indicates when the sorbent has lost its alkalinity. The sorbents are the most effective when they form a barrier between the emission source (wood) and the objects. However, direct contact between object and strong base and charcoal should be avoided.
References
Brokerhof, A.W. and van Bommel, M. (1996) 'Deterioration of calcareous materials by acetic acid vapour: a model study'; in ICOM-CC Preprints of the 11th Triennial Meeting, Edinburgh, pp. 769-776.
Tetreault, J. Sirois, J. and Stamatopoulou, E. (1998) 'Studies of lead corrosion in acetic acid environments'; Studies in Conservation, 43:17-32.
Table 1. Experimental design and results of the two experiments with eggshells exposed to 100 ppm acetic acid (HAc) at 70-80% RH in the absence and presence of different sorbents. Cumulative weight change (dW) during 8 months exposure and calculated rate of degradation (slope).
Sorbent | RH | [HAc] | Base | Change | dW | Exposure | Slope |
| (%) | (ppm) | (meq) | (m-1) | (%) | (m) | (%/m) | |
| experiment 1 | |||||||
| control | 50 | 0 | 0 | 0 | 0.0 | 8 | 0.0 |
| zero exposure | 70-80 | 0 | 0 | 1 | 0.1 | 8 | 0.0 |
| blank exposure | 70-80 | 100 | 0 | 1 | 30.4 | 6 | 5.1 |
| 10 Copy | 70-80 | 100 | 11 | 1 | 17.9 | 6 | 3.0 |
| 10 KOH filter | 70-80 | 100 | 10 | 1 | 3.8 | 8 | 0.5 |
| 1 Vokes | 70-80 | 100 | 1.6 | 1 | 8.8 | 8 | 1.1 |
| 2 Vokes | 70-80 | 100 | 3.1 | 1 | 3.9 | 8 | 0.5 |
| experiment 2 | |||||||
| control | 50 | 0 | 0 | 0 | 0.0 | 8 | 0.0 |
| blank exposure | 70-80 | 100 | 0 | 1 | 38.9 | 8 | 4.9 |
| 18 Copy | 70-80 | 100 | 20 | 1 | 7.5 | 8 | 0.9 |
| 1 Vokes | 70-80 | 100 | 1.6 | 0 | 1.8 | 1.5 | 1.2 |
| 7.4 | 2.5 | 2.9 | |||||
| 18.4 | 4 | 4.6 | |||||
| 2 Vokes | 70-80 | 100 | 3.2 | 0 | 3.0 | 3 | 1.0 |
| 11.6 | 4 | 2.9 | |||||
| 6.3 | 1 | 6.3 | |||||
| Zeolite | 70-80 | 100 | 0 | 1 | 14.7 | 8 | 1.8 |
| Zeolite | 70-80 | 100 | 0 | 0 | 2.6 | 1.5 | 1.7 |
| 19.1 | 6.5 | 2.9 | |||||
| MicroChamber | 70-80 | 100 | ? | 0 | 1.4 | 1 | 1.4 |
| 2.5 | 1 | 2.5 | |||||
| 7.3 | 2 | 3.7 | |||||
| 20.3 | 4 | 5.1 |
Figure 1. Experimental design for exposure of eggshells to 100ppm acetic acid at 70-80% RH

Figure 2. Experiment 1: cumulative weight change (%) during exposure of eggshells to 100ppm acetic acid at 70-80% RH. Sorbents changed each month
= blank;
= 10x copy paper;
= 1x Vokes;
= 2x Vokes;
= KOH filter paper; X = control

Figure 3. Experiment 2: cumulative weight change (%) during exposure of eggshells to 100ppm acetic acid at 70-80% RH. Sorbents changed each month (c) or not changed (nc)
= blank;
= MicroChamber (nc);
= 1x Vokes;
= zeolite (nc);
= 2x Vokes;
= zeolite (c);
= 18x copy paper (c);
X = control
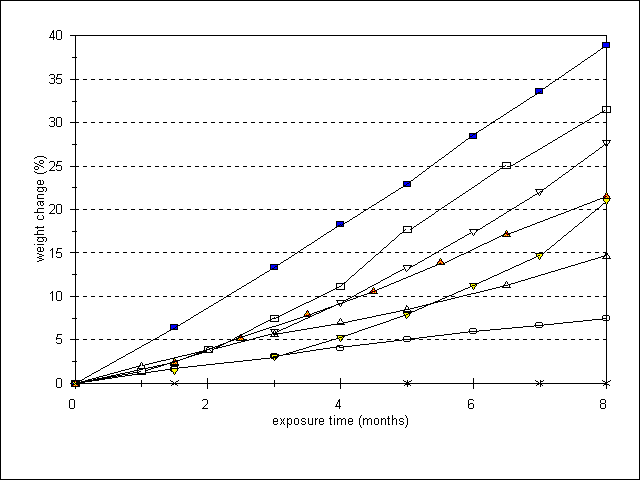
Figure 4. Relative humidity (%RH) in jars during one months exposure with various acid sorbents. Sorbents introduced at day 0, removed at day 26
= 2x Vokes;
= 18x copy paper;
= 10x KOH paper;
= MicroChamber;
= control;
= zeolite (c);
= 1x Vokes
Studies have been undertaken to determine the sorption of acetic acid in cabinets. The question was asked 'Does an acceptable damage concentration exist ?' This would be a concentration below which the reaction takes place so slowly that it is acceptable. Jean has worked with metals (results are in "Studies..") and Agnes has studied calcareous materials. To do this the weight increase of shells and metals were recorded when exposed to different, but known, concentrations of acetic acid vapour. They found that you do see a large weight increase at a certain concentration, indicating that there may be a practical permissible level. RH was found to play a very important role in the corrosion. At less than 40 % RH the speed of corrosion is reduced considerably.
Preventive Conservation options :
Reducing the RH is not always possible. Not all cabinets can be removed, especially if they are part of the collection. Therefore the acid concentration must be reduced to below the agreed permissible level.
In practical lab experiments tried various sorbents in 100 ppm of acetic acid at 80 - 90 % RH : CaCO3 paper lined material, HVAC filter - activated charcoal and KOH, filter paper impregnated with KOH. The weight increase was recorded over time. In the first experiment the weight increase found was : Blank > copy paper > voxes filter > paper + KOH. In the second experiment the weight increase was measured and it was found that blank > microchamber paper > 1 voxes filter > zeolite > 2 voxes filters > zeolite 2 > copy paper.
From the experiments it was also ascertained that the sorbents do release acids after saturation.
[ Page up ] [ IAP Group homepage ] [ Main IAQ in Museums homepage ] [ Search site ]
Indoor Air Quality in Museums and Archives
© April 25th, 2000